- October 20, 2022
- No Comment
- 9 minutes read
Novel PET imaging agent detects earliest signs of Alzheimer's disease – EurekAlert
Society of Nuclear Medicine and Molecular Imaging
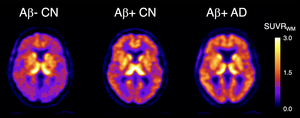
image: 18F-SMBT-1 PET studies showed that beta-amyloid+ Alzheimer’s disease (AD) patients, but most importantly, beta-amyloid+ controls (CN) have significantly higher regional 18F-SMBT-1 binding than beta-amyloid- CN, with 18F-SMBT-1 retention highly associated with beta-amyloid burden. These findings suggest that increased 18F-SMBT-1 binding is detectable at the preclinical stages of beta-amyloid accumulation. view more
Credit: Image created by Victor Villemagne, MD, Professor of Psychiatry at the University of Pittsburgh.
Reston, VA—A new highly selective PET imaging agent can detect the presence of overexpressed monoamine oxidase-B (MAO-B) in cognitively unimpaired individuals with high beta amyloid (Aβ)—one of the earliest signs of Alzheimer’s disease—according to research published in the October issue of The Journal of Nuclear Medicine. The radiotracer, 18F-SMBT-1, allows for a better understanding of the role of inflammation in Alzheimer’s disease, which can enable more accurate staging and prognosis at earlier stages.
Brain inflammation that accompanies Alzheimer’s disease involves reactive astrocytes, which are cells that overexpress MAO-B. The newly developed 18F-SMBT-1 radiotracer is highly selective for MAO-B and as a result has increased binding to reactive astrocytes. “This increased binding suggests that 18F-SMBT-1 can potentially be used as a surrogate marker to detect reactive astrogliosis in Alzheimer’s disease,” noted Victor Villemagne, MD, professor of psychiatry at the University of Pittsburgh in Pittsburgh, Pennsylvania.
The study aimed to characterize 18F-SMBT-1 binding to reactive astrocytes across the Alzheimer’s disease continuum. Study participants included three clinical groups: 57 cognitively unimpaired controls, 12 subjects meeting criteria for mild cognitive impairment (MCI), and eight subjects meeting criteria for Alzheimer’s disease.
Participants underwent several types of imaging, including 18F-SMBT-1 PET, Aβ PET, tau PET, and MRI. Images were normalized and statistical analyses conducted to assess 18F-SMBT-1 binding in relation to Aβ and tau pathology burden. 18F-SMBT-1 was found to be highly correlated with Aβ burden, and much less with tau burden.
The three clinical groups were then classified based on their Aβ status (either as Aβ+ or Aβ-). No significant differences in 18F-SMBT-1 binding were found among Aβ- participants in the control and MCI groups. In the Aβ+ subjects with Alzheimer’s disease, 18F-SMBT-1 binding was significantly higher. Most importantly, 18F-SMBT-1 binding was significantly higher in the Aβ+ control group as compared to Aβ- control group.
“It’s of note that the brain regions where we saw this higher 18F-SMBT-1 binding in the control group are regions known for early Aβ deposition. This suggests that reactive astrocytes are associated with early Aβ deposition at the preclinical stages of Alzheimer’s disease and likely play a role over clinical progression,” said Villemagne.
He continued, “Implementation of 18F-SMBT-1 will clarify the role of reactive astrogliosis in neurodegenerative conditions, not just Alzheimer’s disease and its potential independent and/or synergistic effects on pathology, neurodegeneration, cognition, and disease progression. This has the potential to define and refine the diagnostic, staging and prognostic roles of reactive astrogliosis in these conditions.”
The authors of “Assessing reactive astrogliosis with 18F-SMBT-1 across the Alzheimer’s disease spectrum” include Victor L. Villemagne, Department of Molecular Imaging & Therapy, Austin Health, Melbourne, Australia, and Department of Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania; Ryuichi Harada, Department of Pharmacology, Tohoku University School of Medicine, Sendai, Japan, and Institute of Development of Aging and Cancer, Tohoku University, Sendai, Japan; Vincent Doré, Department of Molecular Imaging & Therapy, Austin Health, Melbourne, Australia, and CSIRO Health and Biosecurity Flagship: The Australian e-Health Research Centre, Melbourne, Australia; Shozo Furumoto, Cyclotron and Radioisotope Center, Tohoku University, Sendai, Japan; Rachel Mulligan, Natasha Krishnadas, Svetlana Bozinovski, and Kun Huang, Department of Molecular Imaging & Therapy, Austin Health, Melbourne, Australia; Yukitsuka Kudo, Institute of Development of Aging and Cancer, Tohoku University, Sendai, Japan; Samantha Burnham, CSIRO Health and Biosecurity Flagship: The Australian e-Health Research Centre, Melbourne, Australia; Pierrick Bourgeat, Ying Xia, and Jürgen Fripp, CSIRO The Australian e-Health Research Centre, Brisbane, Australia; Simon Laws, School of Medical and Health Sciences, Edith Cowan University, Perth, Australia; Milos D. Ikonomovic, Department of Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania, Department of Neurology, University of Pittsburgh, Pittsburgh, Pennsylvania, and Geriatric Research Education and Clinical Center, VA Pittsburgh Healthcare System, Pittsburgh, Pennsylvania; Kazuhiko Yanai, Department of Pharmacology, Tohoku University School of Medicine, Sendai, Japan; Nobuyuki Okamura, Division of Pharmacology, Faculty of Medicine, Tohoku Medical and Pharmaceutical University, Sendai, Japan; and Christopher C. Rowe, Department of Molecular Imaging & Therapy, Austin Health, Melbourne, Australia, The Florey Institute of Neurosciences and Mental Health, The University of Melbourne, Melbourne, Australia, and The Australian Dementia Network.
Visit the JNM website for the latest research, and follow our new Twitter and Facebook pages @JournalofNucMed or follow us on LinkedIn.
###
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected].
About JNM and the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed 15 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.snmjournals.org.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging—precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit www.snmmi.org.
Journal of Nuclear Medicine
10.2967/jnumed.121.263255
Assessing reactive astrogliosis with 18F-SMBT-1 across the Alzheimer’s disease spectrum
3-Oct-2022
Yukitsuka Kudo and Nobuyuki Okamura own stock in Clino Ltd., licensing SMBT-1. Ryuichi Harada, Shozo Furumoto, Yukitsuka Kudo, and Nobuyuki Okamura have a patent pending for the technology described in this article.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
Media Contact
Rebecca Maxey
Society of Nuclear Medicine and Molecular Imaging
[email protected]
Office: 703-652-6772
Society of Nuclear Medicine and Molecular Imaging


Copyright © 2022 by the American Association for the Advancement of Science (AAAS)
Copyright © 2022 by the American Association for the Advancement of Science (AAAS)

